Prior Trial
|
<PROTOCOL TITLE>
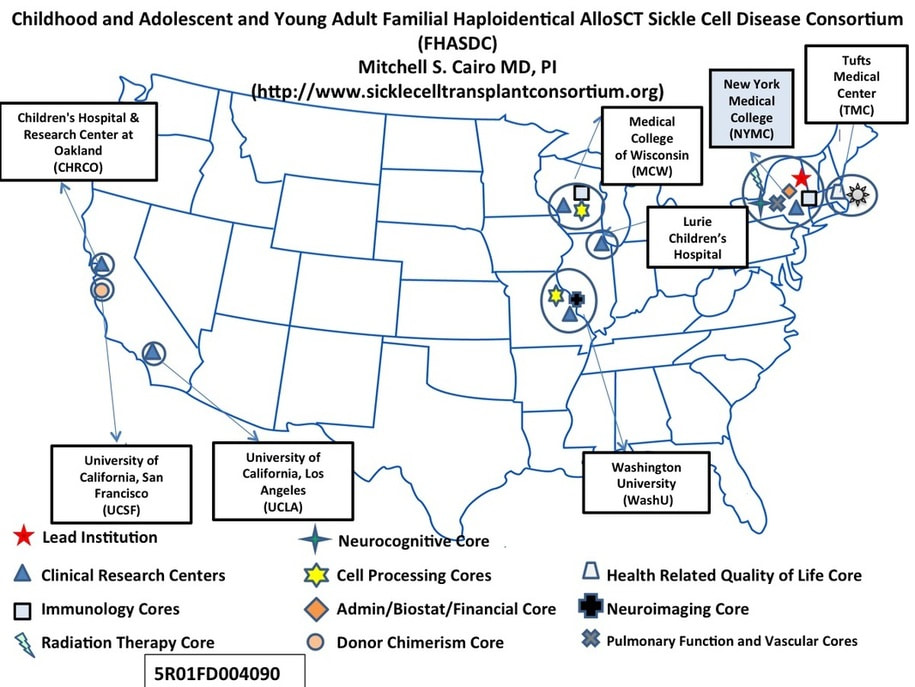
PRIOR COLLABORATING SITES
What was it about?
|
• Lorem ipsum dolor sit amet
• Consectetur adipiscing elit • Lorem ipsum dolor sit amet • Consectetur adipiscing elit |
RESULTS• Lorem ipsum dolor sit amet
• Consectetur adipiscing elit • Lorem ipsum dolor sit amet • Consectetur adipiscing elit |
• Lorem ipsum dolor sit amet
• Consectetur adipiscing elit • Lorem ipsum dolor sit amet • Consectetur adipiscing elit |